The Efficiency Evaluation of Applying Poly Ferric Sulfate and Perlite for Filtration- Juniper Publishers
Juniper Publishers- Journal of Civil Engineering
Abstract
The horrible quality of water from sand filters leads
us to study on the basis and sieving for this sort of filtration. In
addition, we assessed surface charges in the style of 4 samples
regarding chemical treatment from two areas before going to water
treatment plant and from output.
Our inspection demonstrated that the combination of perlite and certain
dosage of poly ferric sulfate with the filter surface 60% of surface
charge shows desirable efficiency.
Keywords: Ferric; Perlite; Turbidity; Liquor; UrbanaizationAbbrevations: Al: Aluminum; Fe: Iron; PACI: Poly Aluminum Chloride; DOC: Dissolved Organic Carbon
Introduction
Growing population, improving of living standards,
urbanization, industrial development and agriculture are the main
factors that increased water consumption and waste water production in
the community, consequently giving rise to environmental pollution [1,2].
Filtration is a separation process that consists in
passing a solid-liquid mixture through a porous material (filter) which
retains the solids and allows the liquid to pass through [3,4].
Removing suspended solids by high-rate granular filtration is a complex
process involving a number of phenomena. Attempts to develop theories
that quantitatively predict solids removal performance with sufficient
precision and versatility to be of use in practical filter design have
met with relatively little success. Consequently, filter media selection
is often an empirical process. Pilot investigations are common tools
for assessing the performance of a particular filter design [5-7].
The used process for water treatment depends on the
quality of water resource. Surface water normally has more variable
pollutants compared with underground water, despite the fact this
surface water could do with more complicated treatment process [8,9].
The most surface water in the world has more pollutants and turbidity
compared with standards for potable water. Although, high velocity of
water stream might have more suspended solids, the most of them are in
colloidal size. Hence, coagulation and filtration are good choice for
treatment [10-12].
The first extensive use of perlite was for filtration of raw cane sugar liquor as early as 1876 [13].
Today the primary industrial application of diatomite is as an
industrial filtration medium for liquids ranging from municipal water
supplies to alcoholic beverages. In contrast, substantial commercial
production of perlite did not begin until 1846. In 1963 only 15 percent
of the perlite produced in the United States was used as filter media [14].
Light weight expanded perlite structures are milled
and classified using strictly defined processes to produce perlite
filter aids with specific flow characteristics. The various grades
utilize the jagged inter locking structure to create billions of
micro-scopic channels between the filter aid particles to produce
optimum flow rates and clarification ability for a wide variety of
applications [15].
Perlite filter aids are light weight, inert, impart
no taste or odor to liquids being filtered and are virtually in soluble
in mineral and organic acids at all temperatures, solubility in strong
alkaline solutions vary depending on temperature and contact time [16].
Without using the filter aids the solid particles in liquid will soon
accumulate on filtering surfaces and block them. A perlite filter aid
makes a filtering layer that transfers the actual filtering from the
septum to the whole mass of filter aid. Filtration occurs in the tiny
pores formed by the fine particles of filter aid [17].
This study aims to find effective factors on
efficiency of filtration in order to enhance efficiency of physical and
chemical parameter and most importantly reducing turbidity [18].
This study presents the recent laboratory research
related to the application of engineered nano particles consisting of in
organic phases in water treatment and their classification in the
fields of heavy metal removal, anti microbial activity and organic
compound degradation. The reported results are discussed not only
according to their potential for application in different drinking water
treatment processes but also from a critical consideration of the
possibility to scale-up in technologically viable methods and become
competitive with existing techniques and conventional materials. As one
of the main limitations in the effort to evaluate the efficiency of nano
materials from different authors is the absence of a unified procedure
that enables direct comparison of results, this work suggests an
experimental methodology working with reliable conditions and parameter
ranges of drinking water treatment and generating proper indices for the
validation of performance.
Aluminum (Al) and iron (Fe) salts are two widely used
coagulants. Large flocs are formed in water sub sequent to hydrolysis
of the metal-based coagulants and then settled in a sedimentation tank.
The floc properties (e.g., size, density and strength) determined by
coagulant species, chemical dosage, and solution chemistry significantly
influence coagulation efficiency. Further, the coagulation performance
can greatly affect the membrane process For example, small floc sizes
lead to high cake resistance on membranes. Prior efforts were made to
determine an optimal coagulant dosage to minimize membrane fouling. Lee
et al. found an optimal poly aluminum chloride (PACl) dosage with
respect to fouling minimization, and the dosage depended heavily on the
physical and chemical characteristics of the waste water. Tran et al.
reported that dissolved organic carbon (DOC) in water could be
significantly reduced with a specified dosage of Al to reduce membrane
fouling.
Methods
Due to the fact that changing materials on the basis
of filtration is much expensive, we assumed the part of it as our model.
The evaluated parameters were turbidity, TSS, total solids, the amount
of Ca and Mg. we got our samples from two area first was the water which
goes to water treatment plant and second was in output of water
treatment plant.
The methods considering the experiments divided into
different stages which each of them implies divergent episodes. First
step was done without any coagulation, we carried out our experiments on
filtration soon after sedimentation and we attained the chemical
properties after our experiments on two samples and we call this step A.
Second step was done with using poly ferric sulfate as our best
coagulants, we used poly ferric sulfate with dosage of 8ppm as the
optimum dose, several hours later of jar test, we implemented filtration
experiment on this sample and attained the results on chemical
properties and we named this step B. Next step was done with combination
of using perlite and anthracite, in this step we added perlite with
anthracite on the surface of filtration, we arranged the array of them
respectively from upside to down part, perlite 48cm (ranging of sieving
0.5-2mm), anthracite 45cm (1-3mm), perlite 15cm (4-6mm), perlite 24cm
(5-8). After making adjustment on grading we performed our experiments
and attained the results and we named it C. The last step was done
similar to prior one but with the difference of height of used materials
in filtration, we changed the surface charge and reduced it up to 60%
of our last experiments, after making some alteration on grading and
surface charges we attained our results and we named it D.
Results
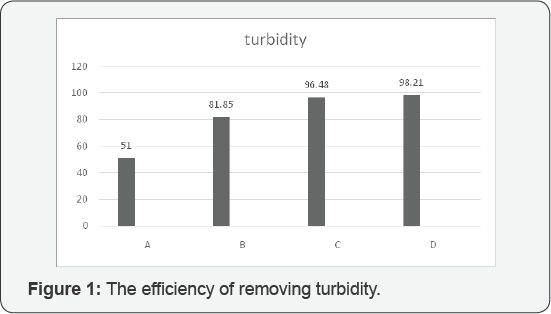
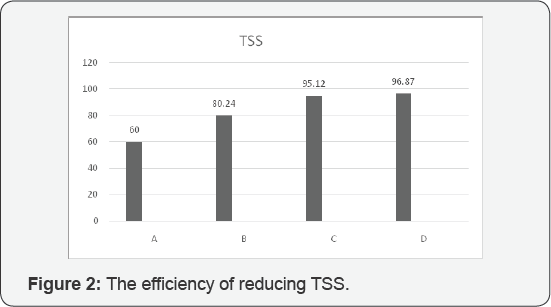
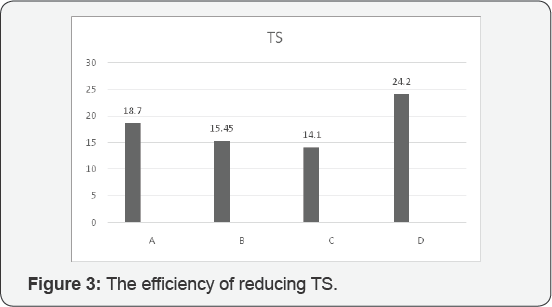
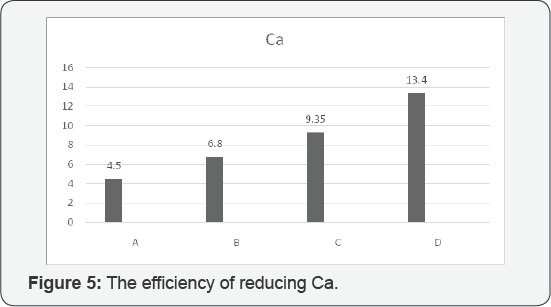
According to our evaluation and analysis of
experiments, these are being claimed that the best efficiency of
turbidity assigned to C and D respectively 96.48% and 98.21%. The best
efficiency for reducing TSS was assigned to D with 96.87 %, moreover,
the best efficiency for reducing total solids was allocated to D with
24.2%, the best efficiency for reducing Ca was assigned to D with 13.4
%. And also fourth stage succeeded to get the best efficiency of
reducing Mg with 26.5 %. (Figure 1-5).

Conclusion
Pursuant to our results in analyzing our samples
considering the parameters TSS, turbidity, total solids, Mg and Ca, it
clearly shows that this method is pretty impeccable if we use it
properly in the case of applying poly ferric sulfate for coagulation, it
gives rise to boost the quality of water as it is depicted in charts as
well as reducing turbidity as well as decreasing the amount of fees for
removing disinfection with considering PH and temperature and another
metals materials in the water. In compared with current methods in
scientific world, this method and results demonstrate the new idea and
method in order to get standard quality according to US EPA.
For More Open Access Journals Please Click on: Juniper Publishers
Fore More Articles Please Visit: Civil Engineering Research Journal


Comments
Post a Comment